Sartorius
Tangential Flow Filtration (TFF) Solutions
Tangential Flow Filtration Systems
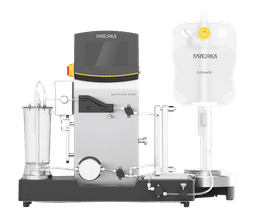
Sartorius offers a range of flexible tangential flow filtration (TFF) systems and consumables for process development and clinical trials, from laboratory environments to commercial production batches. These systems can be configured to accommodate individual requirements, including cost-efficient multi-user systems or closed systems with single-use flow kits for aseptic processing.
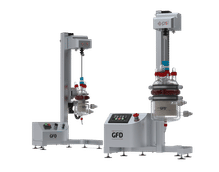
The Sartoflow Smart is a flexible small-scale benchtop TFF system suited for scaling studies and low-volume UF | DF work over a wide range of surface areas, from 50 cm² to as much as 0.14 m².
The Sartoflow Advanced is a highly automated modular benchtop crossflow system optimized for ultrafiltration, microfiltration, and diafiltration. It has two pumps for feed and buffer addition and full TMP control.
Both systems can be used in the downstream purification of vaccines, monoclonal antibodies, and recombinant proteins and provide options to customize the system to your specific requirements. In addition, the corresponding TFF cassettes offer a range of filtration areas and molecular weight cut-offs.
Product classification Tangential Flow Filtration (TFF) Solutions
Product categories
Applications
Manufacturers of similar products
Advertisement